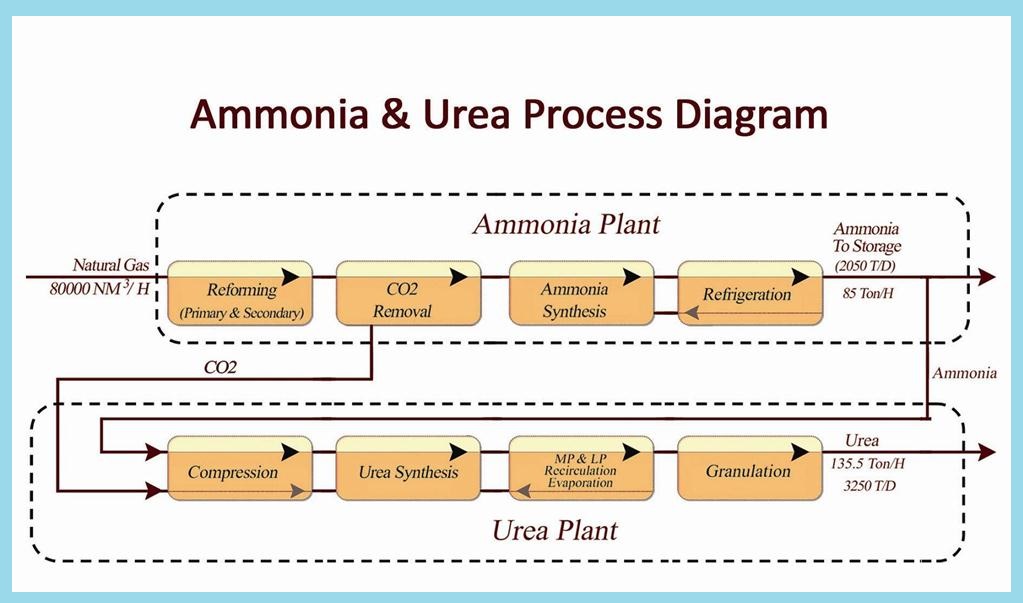
Urea is made from ammonia and carbon dioxide. The ammonia and carbon dioxide are fed into the reactor at high pressure and temperature, and the urea is formed in a two step reaction:
2NH3 + CO2 <=> NH2COONH4 (ammonium carbamate)
NH2COONH4 <=> H2O + NH2CONH2 (urea)
The urea contains unreacted NH3 and CO2 and ammonium carbamate. As the pressure is reduced and heat applied the NH2COONH4 decomposes to NH3 and CO2. The ammonia and carbon dioxide are recycled.
The urea solution is then concentrated to give 99.6% w/w molten urea, and granulated for use as fertilizer and chemical feedstock.
|